
东北大学学报(自然科学版) ›› 2024, Vol. 45 ›› Issue (3): 337-345.DOI: 10.12068/j.issn.1005-3026.2024.03.005
收稿日期:2022-11-07
出版日期:2024-03-15
发布日期:2024-05-17
通讯作者:
路殿坤
作者简介:张 磊(1980-),男,河北石家庄人,东北大学博士研究生,矿冶科技集团有限公司正高级工程师基金资助:
Lei ZHANG1,2, Kai-xi JIANG1,3, Feng XIE1, Dian-kun LU1( )
)
Received:2022-11-07
Online:2024-03-15
Published:2024-05-17
Contact:
Dian-kun LU
About author:LU Dian-kun, E-mail: ludk@smm.neu.edu.cn摘要:
针对刚果金含钴氧化铜精矿采用铁橄榄石渣型进行了焦炭还原熔炼,考察了m(CaO)/m(SiO2),m(FeO)/m(SiO2)和焦比等对铜、钴还原率的影响.对铜、钴、铁和硅氧化物的还原顺序和还原条件进行了热力学分析,借助金属氧化物还原热力学模型与焦炭气化动力学对铜、钴、铁氧化物的还原进程进行了量化分析.研究结果表明,氧化铜精矿中金属铜、钴、铁的还原分为前期的竞争性快速还原期和后期的铜、钴慢速还原期,在慢速还原期已经还原的金属铁还会发生再氧化;金属铁前期大量的竞争性还原和后期的再氧化会降低铜、钴、铁的还原率和焦炭的有效利用率.揭示了粗铜表面铁合金层的形成机制.
中图分类号:
张磊, 蒋开喜, 谢锋, 路殿坤. 氧化铜精矿熔炼过程中铜、钴、铁的还原行为[J]. 东北大学学报(自然科学版), 2024, 45(3): 337-345.
Lei ZHANG, Kai-xi JIANG, Feng XIE, Dian-kun LU. Reduction Behaviors of Copper, Cobalt and Iron in Smelting of Copper Oxide Concentrate[J]. Journal of Northeastern University(Natural Science), 2024, 45(3): 337-345.
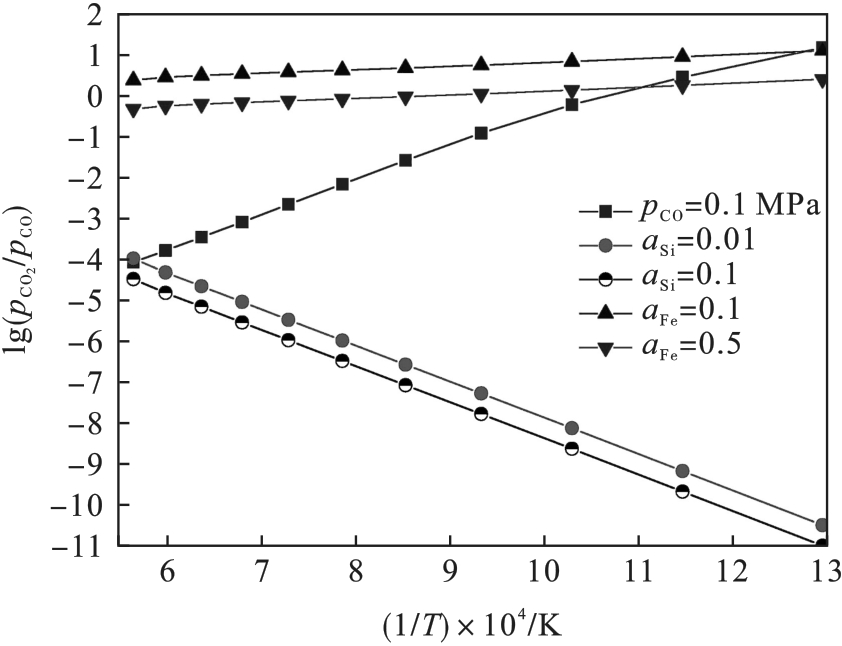
图2 在较低铁、硅活度下FeO和SiO2还原所需的pCO2/pCO比值与温度的关系
Fig.2 Relationships between pCO2/pCO ratios required for the reduction of FeO and SiO2 and temperatures under low iron and silicon activities
| 温度 | Cu2O+CO(g)=2Cu+CO2(g) | CoO+CO(g)=Co+CO2(g) | FeO+CO(g)=Fe+CO2(g) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ℃ | ||||||||||
| 1 273 | 3.99×106 | 1.00 | 1.00 | 13.27 | 0.93 | 1.08 | 0.428 | 0.30 | 3.34 | |
| 1 373 | 1.41×106 | 1.00 | 1.00 | 9.58 | 0.91 | 1.10 | 0.383 | 0.28 | 3.61 | |
| 1 473 | 5.68×105 | 1.00 | 1.00 | 7.29 | 0.88 | 1.14 | 0.348 | 0.26 | 3.87 | |
| 1 573 | 2.15×105 | 1.00 | 1.00 | 5.75 | 0.85 | 1.17 | 0.319 | 0.24 | 4.13 | |
| 1 673 | 9.16×104 | 1.00 | 1.00 | 4.66 | 0.82 | 1.21 | 0.288 | 0.22 | 4.47 | |
| 1 773 | 4.28×104 | 1.00 | 1.00 | 3.89 | 0.80 | 1.26 | 0.244 | 0.20 | 5.10 | |
表2 标准状态下MO还原所需的CO剂量系数与温度之间的关系
Table 2 Relationship between CO coefficient required by MO and temperatures under standard state
| 温度 | Cu2O+CO(g)=2Cu+CO2(g) | CoO+CO(g)=Co+CO2(g) | FeO+CO(g)=Fe+CO2(g) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ℃ | ||||||||||
| 1 273 | 3.99×106 | 1.00 | 1.00 | 13.27 | 0.93 | 1.08 | 0.428 | 0.30 | 3.34 | |
| 1 373 | 1.41×106 | 1.00 | 1.00 | 9.58 | 0.91 | 1.10 | 0.383 | 0.28 | 3.61 | |
| 1 473 | 5.68×105 | 1.00 | 1.00 | 7.29 | 0.88 | 1.14 | 0.348 | 0.26 | 3.87 | |
| 1 573 | 2.15×105 | 1.00 | 1.00 | 5.75 | 0.85 | 1.17 | 0.319 | 0.24 | 4.13 | |
| 1 673 | 9.16×104 | 1.00 | 1.00 | 4.66 | 0.82 | 1.21 | 0.288 | 0.22 | 4.47 | |
| 1 773 | 4.28×104 | 1.00 | 1.00 | 3.89 | 0.80 | 1.26 | 0.244 | 0.20 | 5.10 | |
| Cu | Co | S | FeO | CaO | MgO | Al2O3 | SiO2 |
|---|---|---|---|---|---|---|---|
| 20.92 | 0.23 | 0.82 | 1.94 | 2.91 | 5.99 | 4.65 | 39.08 |
表3 氧化铜精矿主要成分(质量分数) ((mass fraction) %)
Table 3 Main components of copper oxide concentrate
| Cu | Co | S | FeO | CaO | MgO | Al2O3 | SiO2 |
|---|---|---|---|---|---|---|---|
| 20.92 | 0.23 | 0.82 | 1.94 | 2.91 | 5.99 | 4.65 | 39.08 |
| m(CaO)/m(SiO2) | 还原率/% | 产率/% | |||||||
|---|---|---|---|---|---|---|---|---|---|
| g·g-1 | Cu | Co | Fe | 金属 | 炉渣 | ||||
| 0.2 | 99.39 | 98.38 | 54.54 | 1.28 | 1.39 | 32.60 | 74.47 | ||
| 0.3 | 99.59 | 98.30 | 60.27 | 1.41 | 1.60 | 32.33 | 78.07 | ||
| 0.4 | 99.00 | 98.31 | 55.46 | 1.30 | 1.42 | 37.13 | 77.60 | ||
| 0.5 | 99.38 | 98.11 | 43.73 | 1.02 | 1.03 | 31.53 | 86.87 | ||
| 0.6 | 99.56 | 97.99 | 39.04 | 0.91 | 0.89 | 29.13 | 92.40 | ||
| 0.7 | 99.07 | 96.62 | 41.47 | 0.97 | 0.96 | 28.80 | 97.07 | ||
表 4 m(FeO)/m(SiO2)为0.71时m(CaO)/ m(SiO2)对还原熔炼指标的影响
Table 4 Effect of m(CaO)/m(SiO2) on the reduction smelting index with m(FeO)/m(SiO2)= 0.71
| m(CaO)/m(SiO2) | 还原率/% | 产率/% | |||||||
|---|---|---|---|---|---|---|---|---|---|
| g·g-1 | Cu | Co | Fe | 金属 | 炉渣 | ||||
| 0.2 | 99.39 | 98.38 | 54.54 | 1.28 | 1.39 | 32.60 | 74.47 | ||
| 0.3 | 99.59 | 98.30 | 60.27 | 1.41 | 1.60 | 32.33 | 78.07 | ||
| 0.4 | 99.00 | 98.31 | 55.46 | 1.30 | 1.42 | 37.13 | 77.60 | ||
| 0.5 | 99.38 | 98.11 | 43.73 | 1.02 | 1.03 | 31.53 | 86.87 | ||
| 0.6 | 99.56 | 97.99 | 39.04 | 0.91 | 0.89 | 29.13 | 92.40 | ||
| 0.7 | 99.07 | 96.62 | 41.47 | 0.97 | 0.96 | 28.80 | 97.07 | ||
| m(FeO) | 入炉FeO质量/g | a | ||||
|---|---|---|---|---|---|---|
| m(SiO2) | ||||||
| 0.41 | 15.92 | 73.05 | 57.98 | 1.26 | 1.36 | 2.59 |
| 0.56 | 21.91 | 72.82 | 48.25 | 1.51 | 1.77 | 2.16 |
| 0.71 | 27.90 | 55.46 | 42.69 | 1.30 | 1.42 | 1.91 |
| 0.79 | 30.89 | 55.56 | 40.72 | 1.36 | 1.53 | 1.82 |
| 0.87 | 33.89 | 36.19 | 39.10 | 0.93 | 0.91 | 1.75 |
| 0.94 | 36.89 | 37.71 | 37.74 | 1.00 | 1.00 | 1.69 |
| 1.02 | 39.88 | 28.2 | 36.59 | 0.77 | 0.72 | 1.64 |
| 1.10 | 42.87 | 28.96 | 35.60 | 0.81 | 0.77 | 1.59 |
| 1.17 | 45.87 | 19.06 | 34.73 | 0.55 | 0.49 | 1.55 |
| 1.25 | 48.87 | 15.05 | 33.97 | 0.44 | 0.38 | 1.52 |
表5 m(CaO)/m(SiO2)为0.4时m(FeO)/m(SiO2)对还原熔炼指标的影响
Table 5 Effect of m(FeO)/m(SiO2) on the reduction smelting index with m(CaO)/m(SiO2)=0.4
| m(FeO) | 入炉FeO质量/g | a | ||||
|---|---|---|---|---|---|---|
| m(SiO2) | ||||||
| 0.41 | 15.92 | 73.05 | 57.98 | 1.26 | 1.36 | 2.59 |
| 0.56 | 21.91 | 72.82 | 48.25 | 1.51 | 1.77 | 2.16 |
| 0.71 | 27.90 | 55.46 | 42.69 | 1.30 | 1.42 | 1.91 |
| 0.79 | 30.89 | 55.56 | 40.72 | 1.36 | 1.53 | 1.82 |
| 0.87 | 33.89 | 36.19 | 39.10 | 0.93 | 0.91 | 1.75 |
| 0.94 | 36.89 | 37.71 | 37.74 | 1.00 | 1.00 | 1.69 |
| 1.02 | 39.88 | 28.2 | 36.59 | 0.77 | 0.72 | 1.64 |
| 1.10 | 42.87 | 28.96 | 35.60 | 0.81 | 0.77 | 1.59 |
| 1.17 | 45.87 | 19.06 | 34.73 | 0.55 | 0.49 | 1.55 |
| 1.25 | 48.87 | 15.05 | 33.97 | 0.44 | 0.38 | 1.52 |
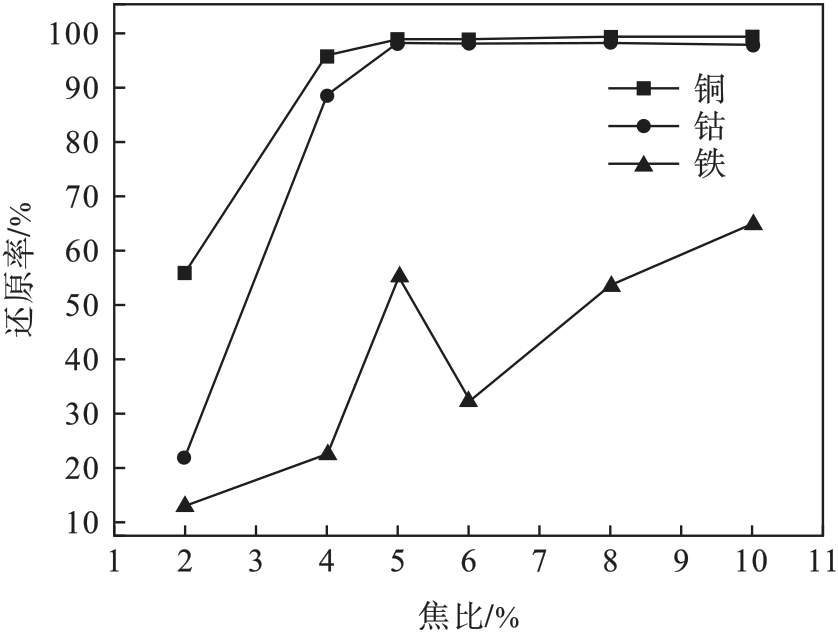
图6 m(FeO)/m(SiO2)为0.71,m(CaO)/m(SiO2)为0.4时焦比对金属还原率的影响
Fig.6 Effect of coke rate on the reduction rate of metals with m(FeO)/m(SiO2)=0.71 and m(CaO)/m(SiO2)=0.4
| 焦比/% | 质量分数/% | n(O)/n(Si) | |||||
|---|---|---|---|---|---|---|---|
| CaO | MgO | Al2O3 | FeO | SiO2 | |||
| 2 | 14.47 | 5.59 | 10.20 | 24.38 | 37.88 | 0.410 | 3.76 |
| 4 | 17.71 | 6.85 | 9.18 | 25.19 | 43.34 | 0.436 | 3.55 |
| 5 | 20.43 | 8.26 | 9.15 | 11.33 | 48.64 | 0.497 | 3.23 |
| 6 | 18.09 | 6.92 | 11.30 | 21.87 | 45.72 | 0.455 | 3.49 |
| 8 | 19.84 | 7.94 | 9.86 | 16.21 | 52.2 | 0.498 | 3.23 |
| 10 | 20.90 | 8.34 | 9.20 | 11.94 | 51.55 | 0.506 | 3.19 |
表6 m(FeO)/m(SiO2)为0.71,m(CaO)/m(SiO2)为0.4时不同焦比下炉渣的成分
Table 6 Slag composition with m(FeO)/m(SiO2)=0.71 and m(CaO)/m(SiO2)=0.4 under various coke rates
| 焦比/% | 质量分数/% | n(O)/n(Si) | |||||
|---|---|---|---|---|---|---|---|
| CaO | MgO | Al2O3 | FeO | SiO2 | |||
| 2 | 14.47 | 5.59 | 10.20 | 24.38 | 37.88 | 0.410 | 3.76 |
| 4 | 17.71 | 6.85 | 9.18 | 25.19 | 43.34 | 0.436 | 3.55 |
| 5 | 20.43 | 8.26 | 9.15 | 11.33 | 48.64 | 0.497 | 3.23 |
| 6 | 18.09 | 6.92 | 11.30 | 21.87 | 45.72 | 0.455 | 3.49 |
| 8 | 19.84 | 7.94 | 9.86 | 16.21 | 52.2 | 0.498 | 3.23 |
| 10 | 20.90 | 8.34 | 9.20 | 11.94 | 51.55 | 0.506 | 3.19 |
| 焦比/% | 焦炭利用率占比/% | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cu | Fe | ||||||||||
| 2 | 12.98 | 18.79 | 0.84 | 0.69 | 0.63 | 66.41 | 18.23 | ||||
| 4 | 22.55 | 34.71 | 1.55 | 0.65 | 0.59 | 56.98 | 15.83 | ||||
| 5 | 55.46 | 42.67 | 1.91 | 1.30 | 1.42 | 47.12 | 31.14 | ||||
| 6 | 32.13 | 50.63 | 2.26 | 0.63 | 0.57 | 39.19 | 15.03 | ||||
| 8 | 53.68 | 66.56 | 2.98 | 0.81 | 0.76 | 29.53 | 18.84 | ||||
| 10 | 64.83 | 82.48 | 3.69 | 0.79 | 0.74 | 23.34 | 18.20 | ||||
表7 m(FeO)/m(SiO2)为0.71,m(CaO)/m(SiO2)为0.4时焦比对铁还原和焦炭利用的影响
Table 7 Effect of coke rate on iron reduction and coke utilization with m(FeO)/m(SiO2)=0.71 and m(CaO)/m(SiO2)=0.4
| 焦比/% | 焦炭利用率占比/% | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cu | Fe | ||||||||||
| 2 | 12.98 | 18.79 | 0.84 | 0.69 | 0.63 | 66.41 | 18.23 | ||||
| 4 | 22.55 | 34.71 | 1.55 | 0.65 | 0.59 | 56.98 | 15.83 | ||||
| 5 | 55.46 | 42.67 | 1.91 | 1.30 | 1.42 | 47.12 | 31.14 | ||||
| 6 | 32.13 | 50.63 | 2.26 | 0.63 | 0.57 | 39.19 | 15.03 | ||||
| 8 | 53.68 | 66.56 | 2.98 | 0.81 | 0.76 | 29.53 | 18.84 | ||||
| 10 | 64.83 | 82.48 | 3.69 | 0.79 | 0.74 | 23.34 | 18.20 | ||||
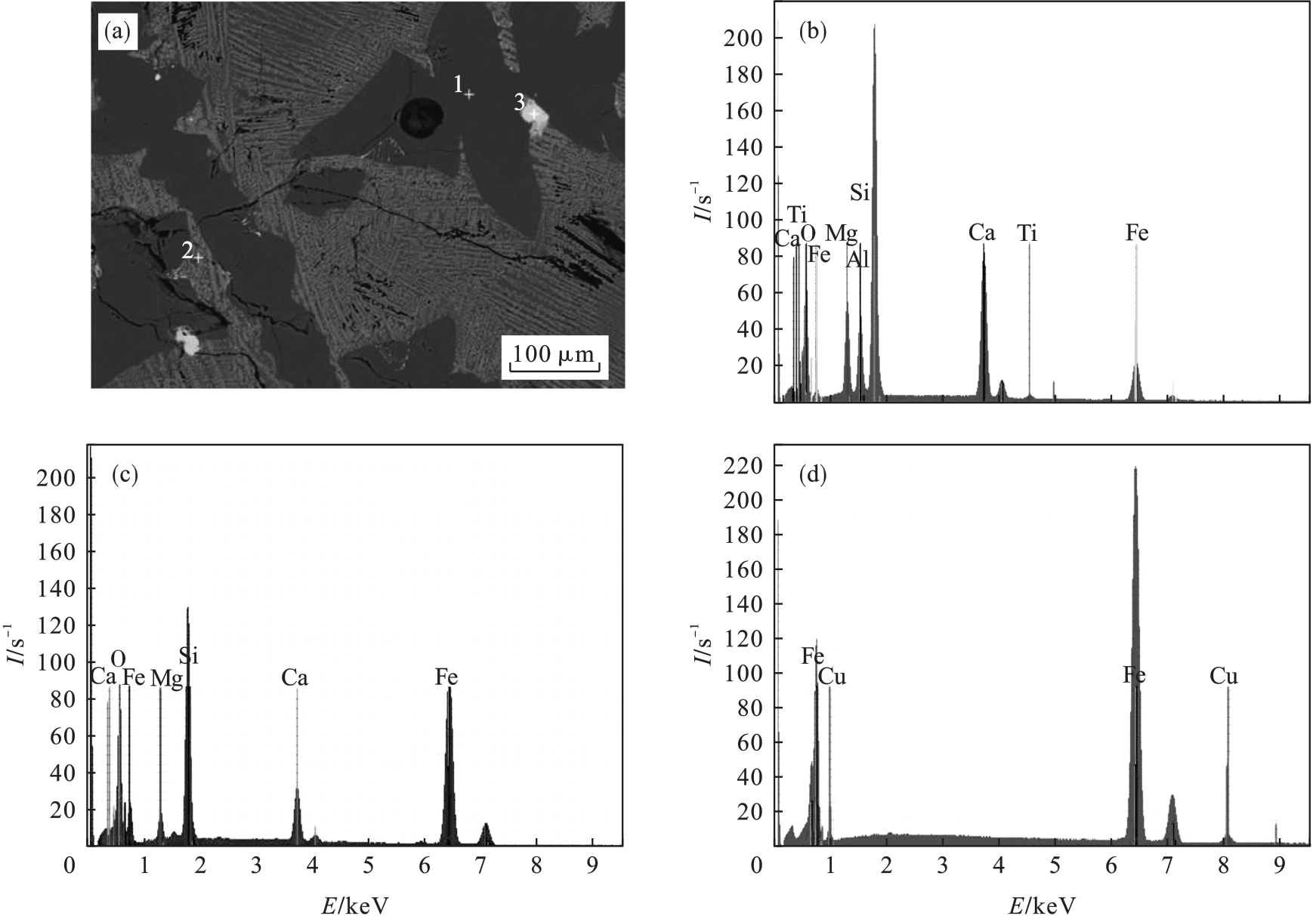
图8 m(FeO)/m(SiO2)为0.71,m(CaO)/m(SiO2)为0.4,焦比为5%时炉渣的SEM+EDS图谱(a)—SEM照片; (b)—谱图1; (c)—谱图2; (d)—谱图3.
Fig.8 SEM+EDS pattern of the slag with m(FeO)/m(SiO2)=0.71, m(CaO)/m(SiO2)=0.4 and coke rate 5%
| 元素 | 谱图1 | 谱图2 | 谱图3 | |||
|---|---|---|---|---|---|---|
质量 分数 | 原子 分数 | 质量 分数 | 原子 分数 | 质量 分数 | 原子 分数 | |
| O | 43.54 | 59.96 | 32.06 | 57.14 | — | — |
| Mg | 7.15 | 6.48 | 0.88 | 1.03 | — | — |
| Al | 6.27 | 5.12 | — | — | — | — |
| Si | 21.63 | 16.97 | 14.07 | 14.29 | — | — |
| Ca | 19.33 | 10.63 | 2.36 | 1.68 | — | — |
| Fe | 1.80 | 0.71 | 50.63 | 25.86 | 85.84 | 87.34 |
| Ti | 0.28 | 0.13 | — | — | — | — |
| Cu | — | — | — | — | 14.16 | 12.66 |
表8 图8中EDS谱图点的元素含量 (%)
Table 8 Element analysis of EDS spectrum points in Fig.8
| 元素 | 谱图1 | 谱图2 | 谱图3 | |||
|---|---|---|---|---|---|---|
质量 分数 | 原子 分数 | 质量 分数 | 原子 分数 | 质量 分数 | 原子 分数 | |
| O | 43.54 | 59.96 | 32.06 | 57.14 | — | — |
| Mg | 7.15 | 6.48 | 0.88 | 1.03 | — | — |
| Al | 6.27 | 5.12 | — | — | — | — |
| Si | 21.63 | 16.97 | 14.07 | 14.29 | — | — |
| Ca | 19.33 | 10.63 | 2.36 | 1.68 | — | — |
| Fe | 1.80 | 0.71 | 50.63 | 25.86 | 85.84 | 87.34 |
| Ti | 0.28 | 0.13 | — | — | — | — |
| Cu | — | — | — | — | 14.16 | 12.66 |
| 1 | Fay I, Barton M D.Alteration and ore distribution in the Proterozoic mines series,Tenke‐Fungurume Cu‐Co district,Democratic Republic of Congo[J].Mineralium Deposita,2012,47(5):501-519. |
| 2 | 周灼刚.用密闭鼓风炉熔炼氧化铜矿方法浅析[J].世界有色金属,2000(9):9-13. |
| Zhou Zhuo‐gang.Analysis on the method of smelting copper oxide in a closed blast furnace[J].World Nonferrous Metals,2000(9):9-13. | |
| 3 | Hauptmann A.The archaeometallurgy of copper [M].Berlin:Springer,2007:217-254. |
| 4 | 苏凤来.降低密闭鼓风炉还原熔炼氧化铜矿炉渣品位的探讨[J].有色金属(冶炼部分),2017,39(9):12-14. |
| Su Feng‐lai.Discussion on reducing grade of copper smelting slag by reduction smelting in closed blast furnace[J].Nonferrous Metals (Extractive Metallurgy),2017,39(9):12-14. | |
| 5 | 陈静,石晓波,龚丽维,等.碱式碳酸铜的制备[J].大学化学,2012,27(5):78-82. |
| Chen Jing, Shi Xiao‐bo, Gong Li‐wei,et al.Preparation of basic copper carbonate[J].University Chemistry,2012,27(5):78-82. | |
| 6 | Li Y, Ren J, Wei H,et al.Reduction kinetics of cold‐bonded briquette prepared from return fines of sinter with carbon monoxide and coke[J].Steel Research International,2023,94(8):1-14. |
| 7 | Turkdogan E T, Vinters J V.Effect of carbon monoxide on the rate of oxidation of charcoal,graphite and coke in carbon dioxide[J].Carbon,1970,8(1):39-53. |
| 8 | Alain V.Extractive metallurgy 2—metallurgical reaction processes[M].London:ISTE Ltd,2011. |
| 9 | Alain V.Extractive metallurgy 1—basic thermodynamics and kinetics [M].London:ISTE Ltd,2011. |
| 10 | ASM International Alloy Phase Diagram and the Handbook Committees.ASM handbook:alloy phase diagrams[M]. Portland:ASM International,1992. |
| 11 | Xiao W B, Yao S W, Zhou S W,et al.Evolution of the structure and viscosity of copper slag during metallization‐reduction[J].Journal of Alloys and Compounds,2022,903:163751. |
| 12 | Li B W, He M S, Huang J Y .Characteristics of anorthite‐pyroxene ceramics made from hot‐poured steelmaking slag[J].JOM,2017,69(2):173-177. |
| 13 | Wu G X, Yazhenskikh E, Hack K,et al.Viscosity model for oxide melts relevant to fuel slags.part 2:the system SiO2-Al2O3-CaO-MgO-Na2O-K2O[J].Fuel Processing Technology,2015,138:520–533. |
| 14 | Hack K, Wu G X, Yazhenskikh E,et al.A CALPHAD approach to modelling of slag viscosities[J].Computer Coupling of Phase Diagrams and Thermochemistry,2019,65:101-110. |
| 15 | 王震,姜茂发,刘承军,等.CaO-SiO2-Al2O3-Na2O-MgO系模铸保护渣微观结构解析[J].东北大学学报(自然科学版),2023,44(4):517-523. |
| Wang Zhen, Jiang Mao‐fa, Liu Cheng‐jun,et al.Microstructure analysis of mold flux of CaO‐SiO2‐Al2O3‐Na2O‑MgO system for ingot casting[J].Journal of Northeastern University(Natural Science),2023,44(4):517-523. |
| [1] | 冯宇庭, 成丽, 王可媚, 胡恩柱. 金属矿物对丁铵黑药在饱和多孔介质中迁移特性的影响[J]. 东北大学学报(自然科学版), 2024, 45(3): 401-406. |
| [2] | 郭谨铭, 杨洪英, 佟琳琳, 孟晶. 西藏某铜钼混合精矿工艺矿物学研究[J]. 东北大学学报(自然科学版), 2023, 44(8): 1195-1200. |
| [3] | 成丽, 梁海军, 南相莉, 胡恩柱. 丁基黄药在金属负载石英砂中的迁移特性[J]. 东北大学学报(自然科学版), 2023, 44(5): 712-718. |
| [4] | 魏作安, 谢金鑫, 赵筠康, 路停. 化学抑尘剂对铜尾矿库区草本植物适生性的影响[J]. 东北大学学报(自然科学版), 2022, 43(12): 1799-1804. |
| [5] | 赵冰, 张冉, 徐新阳. 污泥基磁性生物炭及其对水体中铜离子的吸附性能[J]. 东北大学学报(自然科学版), 2021, 42(7): 1012-1018. |
| [6] | 肖发新, 彭宇, 孙树臣, 涂赣峰. (NH4)2SO4-NH3-H2O体系浸出高碱性脉石低品位氧化铜矿[J]. 东北大学学报(自然科学版), 2021, 42(6): 795-801. |
| [7] | 马英强, 郑双林, 盛秋月, 姚金. 乙二胺磷酸钠对赤铜矿硫化浮选的影响及其机理[J]. 东北大学学报:自然科学版, 2020, 41(7): 1008-1014. |
| [8] | 张培红, 袁威, 魏钟原, 李子建. 湿热环境下NCM三元锂离子电池热失控分析[J]. 东北大学学报:自然科学版, 2020, 41(6): 881-887. |
| [9] | 巩亚东, 孟凡涛, 孙瑶, 于兴晨. 镍基单晶高温合金的微孔加工对比实验研究[J]. 东北大学学报:自然科学版, 2020, 41(1): 84-89. |
| [10] | 吕江涛, 王筱钧, 鄂思宇, 张亚男. 用于硝酸根浓度测量的反射式光纤SPR传感器[J]. 东北大学学报:自然科学版, 2019, 40(8): 1075-1079. |
| [11] | 陈敬琪, 刘相华, 闫述, 于庆波. 铜/钢/铜冷轧复合薄带弯曲性能实验研究[J]. 东北大学学报:自然科学版, 2019, 40(5): 647-652. |
| [12] | 沈玲玲, 赵博, 徐君莉, 石忠宁. 等离子体放电电解制备纳米氧化亚铜[J]. 东北大学学报:自然科学版, 2019, 40(5): 668-672. |
| [13] | 马小刚, 陈良玉, 李杨. 基于响应面法炉腹异型管铜冷却壁的长寿技术优化[J]. 东北大学学报:自然科学版, 2019, 40(5): 710-715. |
| [14] | 孙乾予, 印万忠, 朱张磊,姚金. 丁基钠黄药浮选斑铜矿的吸附热力学和动力学研究[J]. 东北大学学报:自然科学版, 2019, 40(4): 574-579. |
| [15] | 王改荣, 杨洪英, 佟琳琳, 刘媛媛. 赞比亚卢安夏氧化铜矿工艺矿物学研究[J]. 东北大学学报:自然科学版, 2019, 40(3): 350-355. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||