
东北大学学报(自然科学版) ›› 2025, Vol. 46 ›› Issue (3): 28-45.DOI: 10.12068/j.issn.1005-3026.2025.20240182
收稿日期:2024-10-16
出版日期:2025-03-15
发布日期:2025-05-29
通讯作者:
张涛
作者简介:赵 阳(1989—),男,辽宁鞍山人,东北大学副教授,博士生导师基金资助:
Yang ZHAO, Yu-hang WANG, Tao ZHANG( ), Fu-hui WANG
), Fu-hui WANG
Received:2024-10-16
Online:2025-03-15
Published:2025-05-29
Contact:
Tao ZHANG
About author:ZHANG Tao E-mail:zhangtao@mail.neu.edu.cn
摘要:
在半导体制程设备中,在高温、真空、强腐蚀气体及其等离子体耦合作用下,铝合金涂层极易发生失效.在氯基等离子体中,阳极氧化涂层极易被刻蚀去除,Y2O3涂层的刻蚀速率约为阳极氧化涂层的1/50;在氟基等离子体中,阳极氧化涂层和Y2O3涂层均存在氟化物层剥落导致的颗粒问题.通过调节电解液的成分/温度、制备纯铝层可提高阳极氧化涂层的耐蚀性能,提高Y2O3涂层的致密性同样可降低涂层的刻蚀速率,结合远程等离子体清洗技术,避免带电粒子轰击腔室材料,可显著减少反应腔室中颗粒的产生.在刻蚀和薄膜沉积过程中,腔室表面成分发生变化,进而改变等离子体状态,将引发多种工艺缺陷.
中图分类号:
赵阳, 王宇航, 张涛, 王福会. 半导体制程设备铝合金涂层腐蚀失效行为研究进展[J]. 东北大学学报(自然科学版), 2025, 46(3): 28-45.
Yang ZHAO, Yu-hang WANG, Tao ZHANG, Fu-hui WANG. Research Progress on the Corrosion Failure Behavior of Coatings on Aluminum Alloy for Semiconductor Fabrication Equipment[J]. Journal of Northeastern University(Natural Science), 2025, 46(3): 28-45.
| 湿法擦拭流程 | 湿法清洗流程 |
|---|---|
1. 启动腔室清洁 2. 冷却腔室 3. 泄压开腔 4. 湿洁净布擦拭面板 5. 使用N2干燥面板 6. 关闭腔室 7. 加热腔室 8. 腔室检漏 9. 刻蚀/沉积工艺验证 10. 恢复运行 | 1. 启动腔室清洁 2. 冷却腔室 3. 泄压开腔 4. 拆卸腔室部件 5. 在清洗溶液中浸泡腔室部件 6. 烘干腔室部件 7. 更换腔室部件 8. 关闭腔室 9. 加热腔室 10. 腔室检漏 11. 刻蚀/沉积工艺验证 12. 恢复运行 |
表1 湿法擦拭/清洗步骤[14-16]
Table 1 Wet wiping/cleaning procedures[14-16]
| 湿法擦拭流程 | 湿法清洗流程 |
|---|---|
1. 启动腔室清洁 2. 冷却腔室 3. 泄压开腔 4. 湿洁净布擦拭面板 5. 使用N2干燥面板 6. 关闭腔室 7. 加热腔室 8. 腔室检漏 9. 刻蚀/沉积工艺验证 10. 恢复运行 | 1. 启动腔室清洁 2. 冷却腔室 3. 泄压开腔 4. 拆卸腔室部件 5. 在清洗溶液中浸泡腔室部件 6. 烘干腔室部件 7. 更换腔室部件 8. 关闭腔室 9. 加热腔室 10. 腔室检漏 11. 刻蚀/沉积工艺验证 12. 恢复运行 |
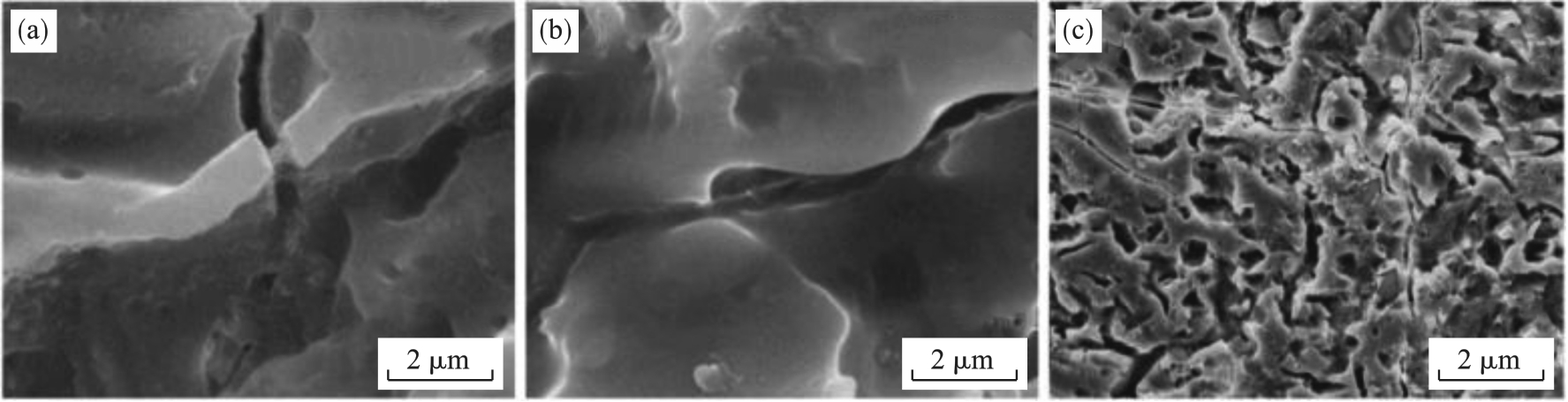
图8 阳极氧化涂层在不同温度下经历热循环测试后的表面形貌[16](a)—120 ℃; (b)—160 ℃; (c)—200 ℃.
Fig. 8 Surface morphology of the anodized coating treated by thermal cycling tests at various temperatures[16]
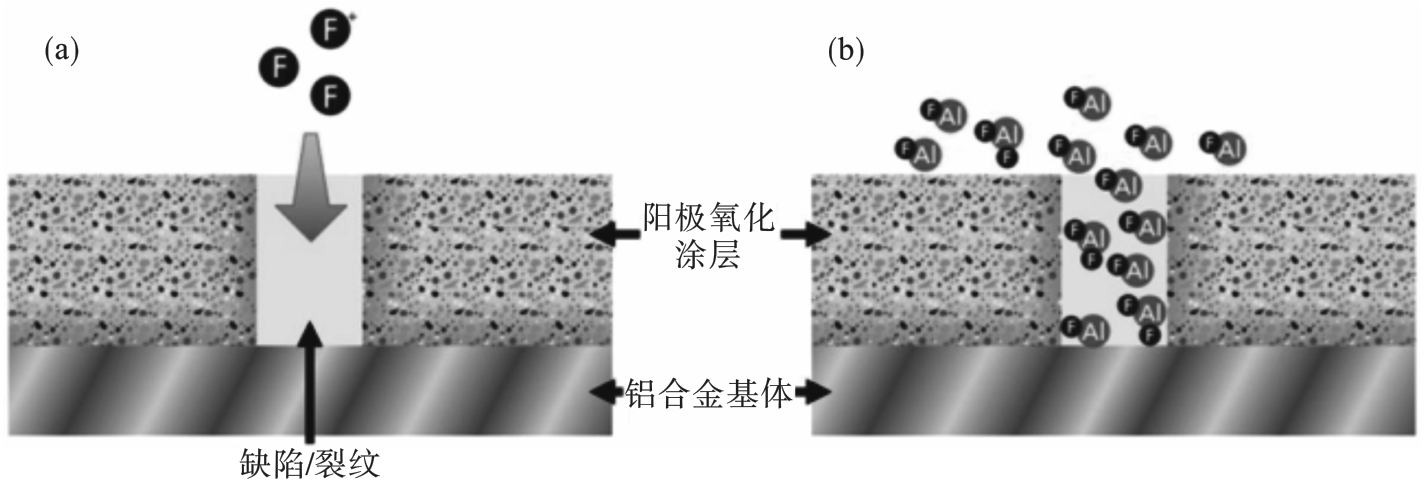
图9 F自由基通过阳极氧化涂层的缺陷/裂纹与铝合金基体反应的截面示意图[17](a)—F自由基穿过涂层; (b)—F自由基与基体反应生成氟化铝.
Fig. 9 Cross-sectional schematic illustrating fluorine radicals penetrating the defects/cracks in the anodized aluminum and reacting with the aluminum substrate[17]
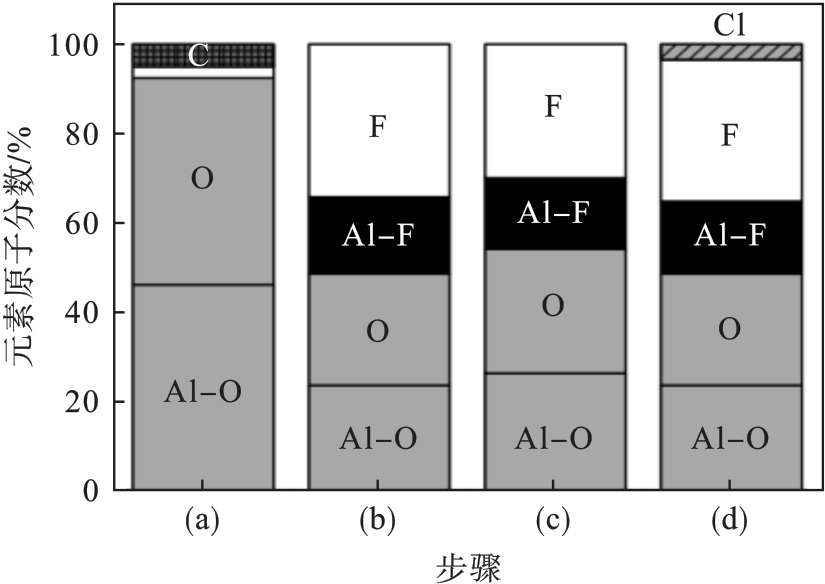
图11 Al2O3试样化学成分的变化[42]注:(a)为清洗前的Al2O3试样; (b)为SF6/O2等离子体清洗,腔室压力为2.66 Pa; (c)为腔室压力为11.31 Pa; (d)为纯Cl2等离子体清洗,腔室压力为1.33 Pa、射频源功率为450 W.
Fig. 11 Chemical composition changes of the Al2O3 sample[42]
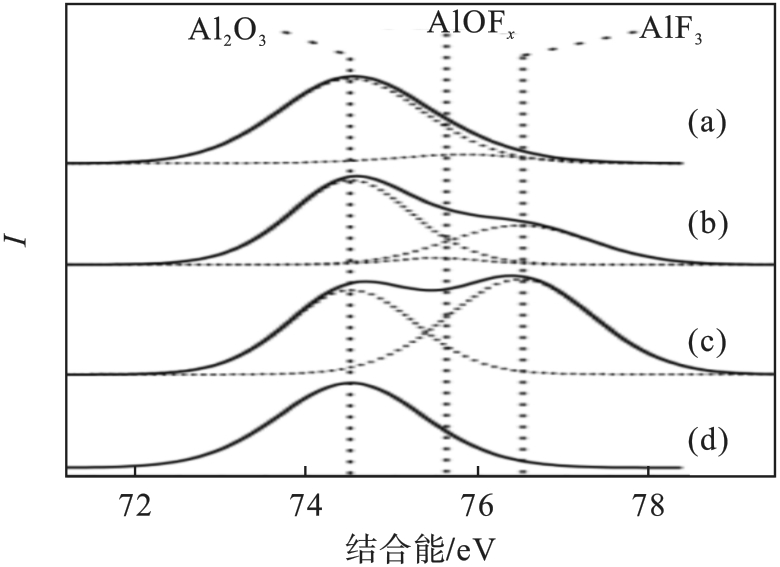
图13 Al2O3试样的Al 2p XPS谱[42]注:(a)为清洗前的Al2O3试样; (b)(c)分别 为SF6/O2等离子体清洗60,300 s; (d)SiCl4/Cl2等离子体清洗20 s.
Fig. 13 Al 2p XPS spectra of the Al2O3 sample [42]
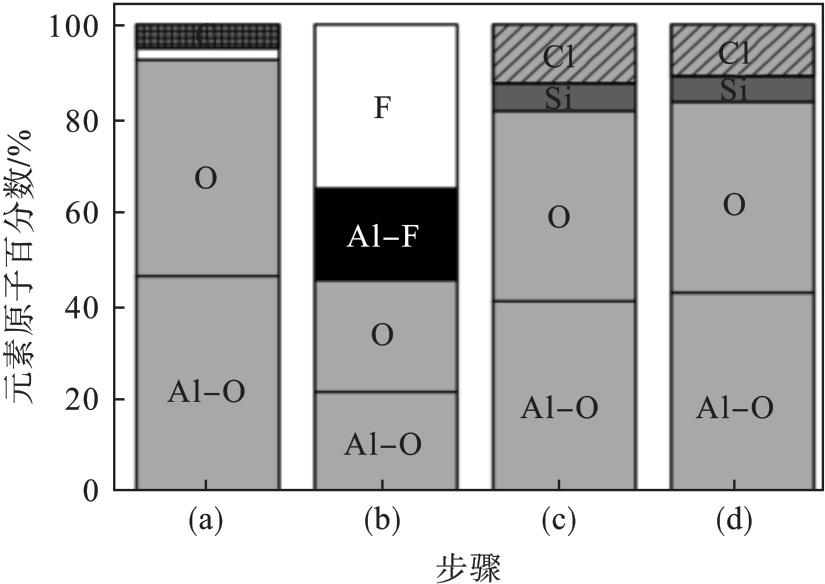
图14 Al2O3 试样化学成分的变化[42].注:(a)为清洗前的Al2O3试样; (b)为SF6/O2等离子体清洗; (c)(d)分别为SiCl4/Cl2等离子体清洗15 s,120 s.
Fig. 14 Chemical composition changes of the Al2O3 sample[42]
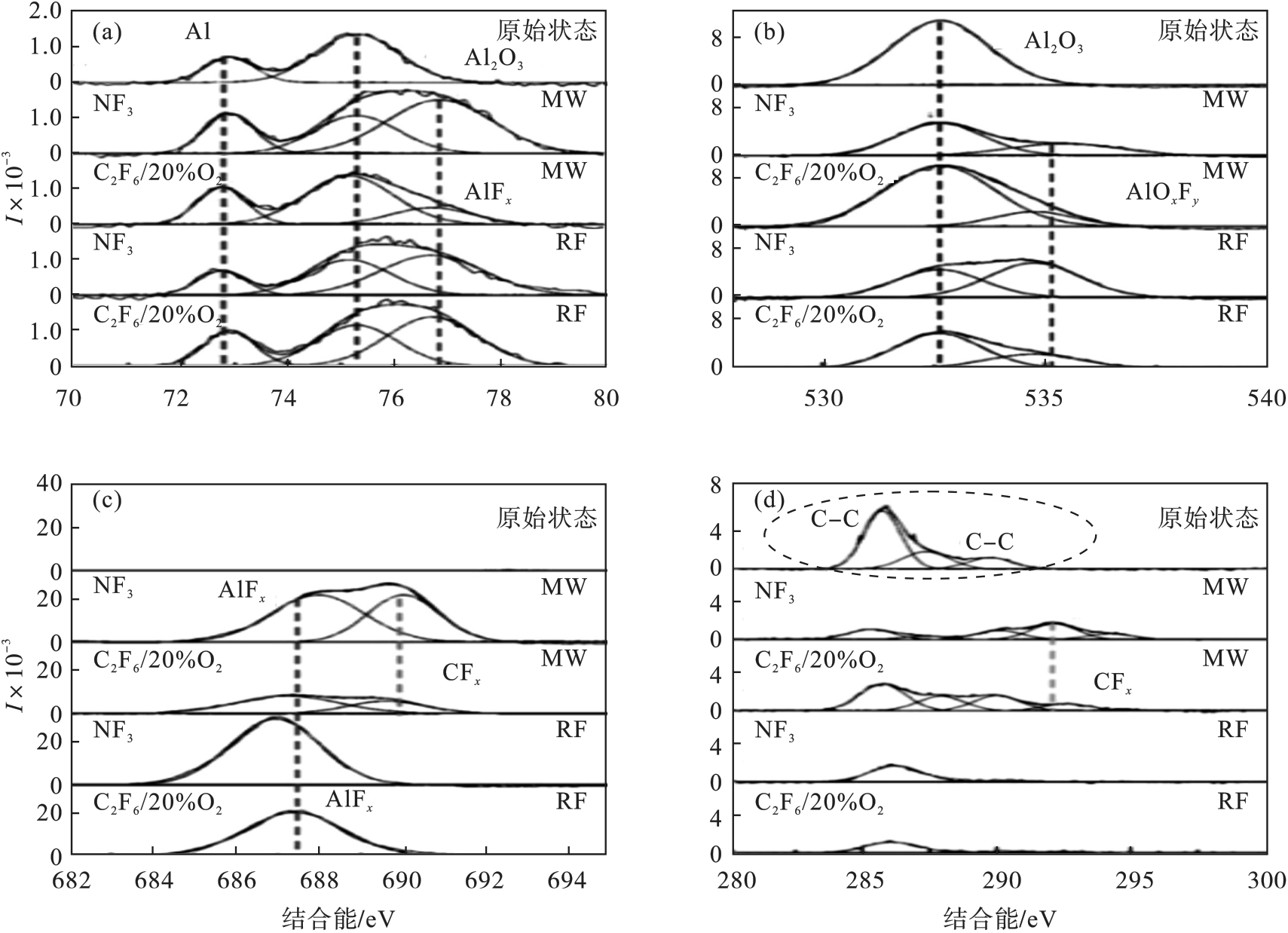
图16 等离子体清洗过程中铝合金样品的化学成分转变[51](a)—Al(2p); (b)—F(1s); (c)—O(1s); (d)—C(1s).
Fig. 16 Chemical composition changes of the aluminum alloy sample during plasma cleaning[51]
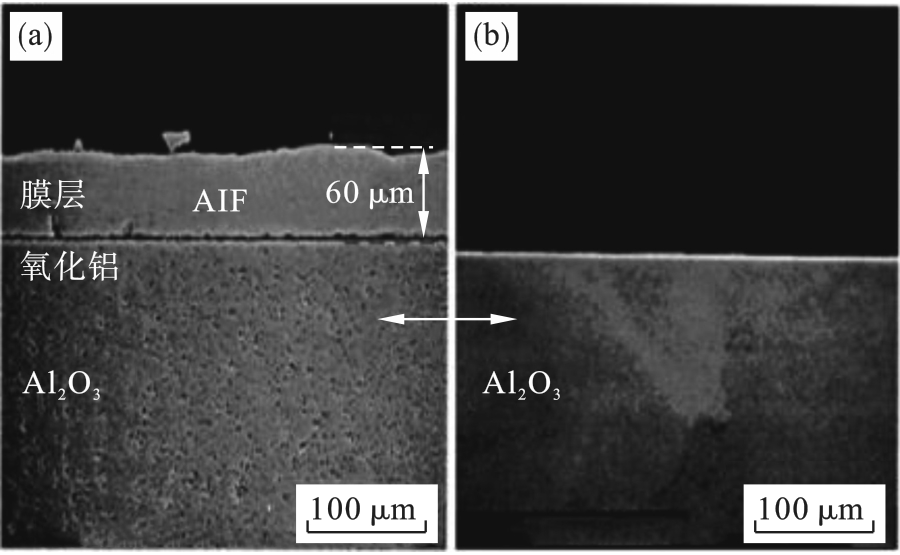
图17 经历150 h NF3等离子体清洗后氧化铝视窗镜的截面形貌[45](a)—原位清洗; (b)—远程微波清洗.
Fig. 17 Cross-sectional morphology of the Al2O3 window after 150 h cleaning by NF3 plasma[45]
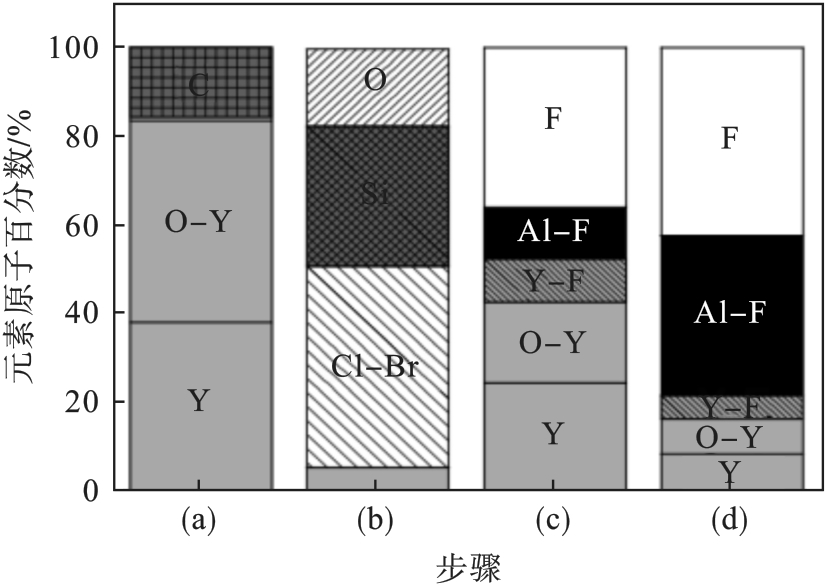
图22 Y2O3试样化学成分的变化[15]注:(a)为清洗前的Y2O3试样;(b)为使用HBr/Cl2/O2等离子体刻蚀硅晶圆后;(c)为SF6/O2等离子体清洗80 s;(d)为SF6/O2等离子体清洗1 200 s.
Fig. 22 Chemical composition changes of the Y2O3 sample[15]
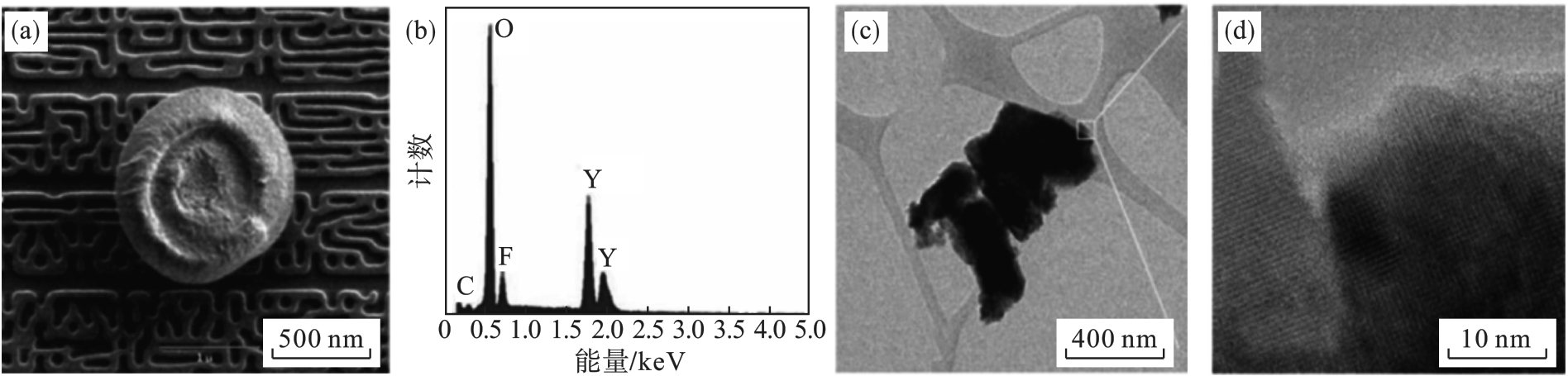
图23 Y2O3涂层表面剥落颗粒的TEM图像及元素分析[63].(a)—颗粒形貌; (b)—颗粒成分; (c)—颗粒形貌; (d)—高分辨图像.
Fig. 23 TEM images and elemental analysis of particles flaked from the Y2O3 coatings [63]
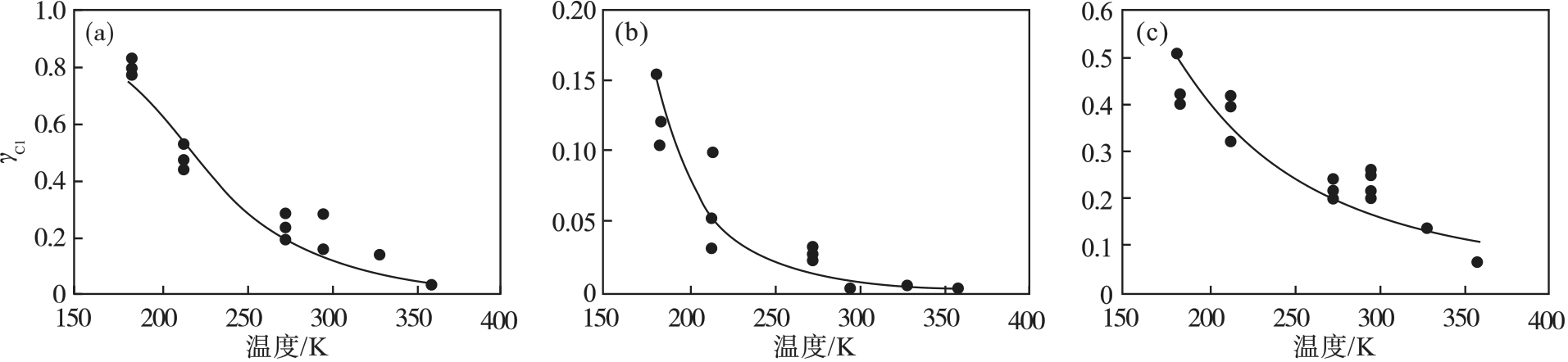
图25 不同材料Cl原子的表面复合系数随温度的变化[72](a)—阳极氧化铝; (b)—石英; (c)—多晶硅.
Fig. 25 Cl atomic surface recombination coefficient as a function of surface temperatures for various materials[72]
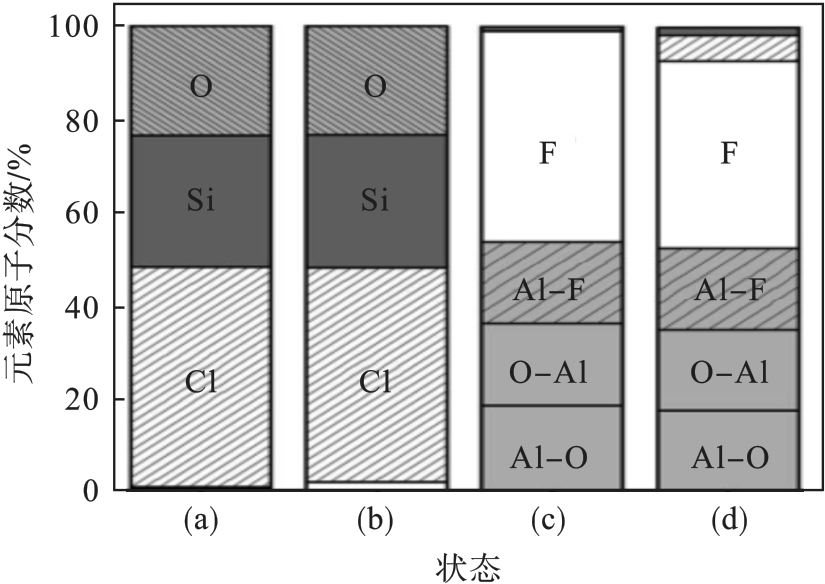
图26 不同表面状态的反应腔室的化学成分[74-75]注:(a~b)为SiO x Cl y 涂层腔室; (c~d)为AlF3涂层腔室.
Fig. 26 Chemical composition of the reactor walls at various surface conditions[74-75]
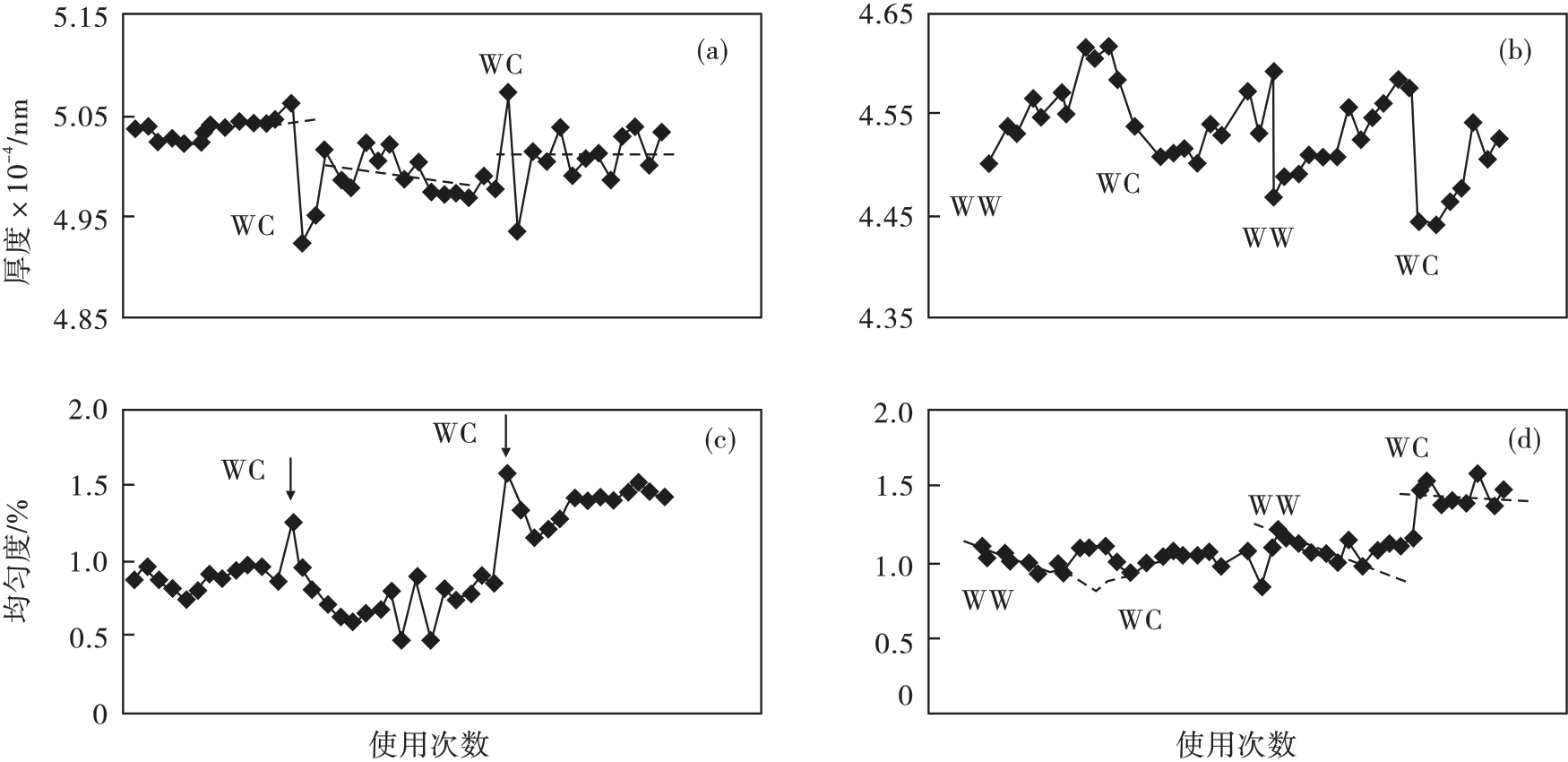
图28 沉积的Si3N4,SiO2膜层厚度、均匀性随腔室使用次数的变化[14](a)—Si3N4膜层厚度; (b)—SiO2膜层厚度; (c)—Si3N4膜层均匀性; (d)—SiO2膜层均匀性.
Fig. 28 Variation in Si3N4 and SiO2 thickness, thickness uniformity with number of chamber use[14]
| 1 | SIA State of industry report 2023[R/OL].Washington, D C (2023). . |
| 2 | Winter R, Korzec D, Engemann J. Remote and direct cleaning by use of microwave plasma source SLAN II: comparative study[J]. Surface and Coatings Technology, 1997, 91(1/2): 101-106. |
| 3 | Manos D M, Flamm D L. Plasma etching: an introduction[M]. Boston: Academic Press, 1989. |
| 4 | Rasgon S A. Origin, evolution, and control of sidewall line edge roughness transfer during plasma etching[D]. Cambridge: Massachusetts Institute of Technology, 2005. |
| 5 | Donnelly V M, Kornblit A. Plasma etching: yesterday, today, and tomorrow[J]. Journal of Vacuum Science & Technology A: Vacuum, Surfaces, and Films, 2013, 31(5): 050825-050873. |
| 6 | Hamedani Y, Macha P, Bunning T J, et al. Plasma-enhanced chemical vapor deposition: where we are and the outlook for the future[M]//Chemical Vapor Deposition-Recent Advances and Applications in Optical, Solar Cells and Solid State Devices. London: InTech,2016. |
| 7 | Armacost M, Hoh P D, Wise R, et al. Plasma-etching processes for ULSI semiconductor circuits[J]. IBM Journal of Research and Development, 1999, 43: 39-72. |
| 8 | Wang Y H, Zhao Y, Wang S G, et al. Thermodynamics-based sealing method for anodized aluminum used in semiconductor processing apparatuses[J]. Journal of Materials Science & Technology, 2025, 216: 241-259. |
| 9 | Ullal S J, Godfrey A R, Edelberg E, et al. Effect of chamber wall conditions on Cl and Cl2 concentrations in an inductively coupled plasma reactor[J]. Journal of Vacuum Science & Technology A: Vacuum, Surfaces, and Films, 2002, 20(1): 43-52. |
| 10 | Kota G P. Experimental beam studies of plasma-surface interactions[D]. Berkeley: University of California, Berkeley, 1998. |
| 11 | Vempaire D, Cunge G. Probing radical kinetics in the afterglow of pulsed discharges by absorption spectroscopy with light emitting diodes: application to BCl radical[J]. Applied Physics Letters, 2009, 94(2): 021504. |
| 12 | Hong S, Lin X, John M K, et al. Dense oxide coated component of a plasma processing chamber and method of manufacture thereof: US9123651B2[P/OL]. 2015[2024-07-10]. . |
| 13 | Daugherty J, Shih H, Xu L, et al. Corrosion resistant aluminum coating on plasma chamber components: US9337002B2[P/OL]. 2016[2024-07-10]. . |
| 14 | Smith B C, Young A. Optimizing chamber cleans for better film deposition performance optimizing chamber cleans for better film deposition performance[J]. Journal of the Electrochemical Society, 2001, 148(11): C721-C727. |
| 15 | Hong S. Corrosion resistance[M]. London: IntechOpen, 2012. |
| 16 | Bourget L, Brucker G, Feaver M, et al. MKS instrument handbook: semiconductor devices and process technology. 2nd ed.[M]. Andover: MKS Instruments, 2023. |
| 17 | Cunge G, Pelissier B, Joubert O, et al. New chamber walls conditioning and cleaning strategies to improve the stability of plasma processes[J]. Plasma Sources Science and Technology, 2005, 14(3): 599-609. |
| 18 | Huang Y L, Shih H, Daugherty J, et al. Evaluation of the properties of anodized aluminum 6061 subjected to thermal cycling treatment using electrochemical impedance spectroscopy (EIS)[J]. Corrosion Science, 2009, 51(10): 2493-2501. |
| 19 | Advanced Energy Industries I. Remote plasma source chamber anodization[R/OL].2018[2024-07-10].. |
| 20 | Raoux S, Cheung D, Fodor M, et al. Growth, trapping and abatement of dielectric particles in PECVD systems[J]. Plasma Sources Science Technology, 1997, 6(3): 405-414. |
| 21 | Tanaka J, Shiraishi K. Evaluation of growth and cleaning rates of chamber-wall deposition during silicon gate etching[J]. E-Journal of Surface Science and Nanotechnology, 2013, 11: 1-7. |
| 22 | Kim M S, Lee J W. Effect of seasoning-layer stress on fluorine diffusion[J]. AIP Advances, 2020, 10(8): 1-8. |
| 23 | Vos M F J. Development and understanding of advanced atomic layer deposition process: AlF3, Co and Ru[D]. Eindhoven: Eindhoven University of Technology, 2019. |
| 24 | Nojiri K. Dry etching technology for semiconductors. 1st ed.[M]. Tokyo: Springer Cham, 2015: 8-12. |
| 25 | Younesy S, Petit-Etienne C, Barnola S, et al. Cleaning chamber walls after ITO plasma etching process[C]// Advanced Etch Technology for Nanopatterning IX. San Jose, 2020: 85-91. |
| 26 | Miwa K, Takada N, Sasaki K. Fluorination mechanisms of Al2O3 and Y2O3 surfaces irradiated by high-density CF4∕O2 and SF6∕O2 plasmas[J]. Journal of Vacuum Science & Technology A: Vacuum, Surfaces, and Films, 2009, 27(4): 831-835. |
| 27 | Padron-Wells G, Vanoverloop M, Yeo J, et al. Fluorine saturated yttrium (YF) based coatings for advanced semiconductor ULSI manufacturing[C]//2019 30th Annual SEMI Advanced Semiconductor Manufacturing Conference (ASMC). Saratoga Springs, 2019: 1-6. |
| 28 | Lee S S, Kim M J, Chung C W, et al. Degradation test for an anodic aluminum oxide film in plasma etching[J]. Journal of the Korean Physical Society, 2019, 74(11): 1046-1051. |
| 29 | Kim D M, Oh Y S, Kim S, et al. The erosion behaviors of Y2O3 and YF3 coatings under fluorocarbon plasma[J]. Thin Solid Films, 2011, 519(20): 6698-6702. |
| 30 | Lutze J W, Perera A H, Krusius J P. Anisotropic reactive ion etching of aluminum using Cl2, BCl3, and CH4 gases[J]. Journal of the Electrochemical Society, 1990, 137(1): 249-252. |
| 31 | Frank W E. Approaches for patterning of aluminum[J]. Microelectronic Engineering, 1997, 33(1/2/3/4): 85-100. |
| 32 | Tokunaga K, Redeker F C, Danner D A, et al. Comparison of aluminum etch rates in carbon tetrachloride and boron trichloride plasmas[J]. Journal of The Electrochemical Society, 1981, 128(4): 851-855. |
| 33 | Keaton A L, Hess D W. Aluminum etching in boron tribromide plasmas[J]. Journal of Vacuum Science & Technology A: Vacuum, Surfaces, and Films, 1985, 3(3): 962-966. |
| 34 | Jeong C H, Kim D W, Lee H Y, et al. Sapphire etching with BCl3/HBr/Ar plasma[J]. Surface and Coatings Technology, 2003, 171(1/2/3): 280-284. |
| 35 | Jeong C H, Kim D W, Bae J W, et al. Dry etching of sapphire substrate for device separation in chlorine-based inductively coupled plasmas[J]. Materials Science and Engineering B: Solid-State Materials for Advanced Technology, 2002, 93(1/2/3): 60-63. |
| 36 | Fukumoto H, Fujikake I, Takao Y, et al. Plasma chemical behaviour of reactants and reaction products during inductively coupled CF4 plasma etching of SiO2 [J]. Plasma Sources Science and Technology, 2009, 18(4): 045027-045044. |
| 37 | Singh V K, Shaqfeh E S G, McVittie J P. Study of silicon etching in CF4/O2 plasmas to establish surface re-emission as the dominant transport mechanism[J]. Journal of Vacuum Science & Technology B: Microelectronics and Nanometer Structures, 1994, 12(5): 2952-2962. |
| 38 | Cunge G, Vempaire D, Ramos R, et al. Radical surface interactions in industrial silicon plasma etch reactors[J]. Plasma Sources Science and Technology, 2010, 19(3): 034017-034028. |
| 39 | Hsueh H P, McGrath R T, Ji B, et al. Ion energy distributions and optical emission spectra in NF3-based process chamber cleaning plasmas[J]. Journal of Vacuum Science & Technology B: Microelectronics and Nanometer Structures, 2001, 19(4): 1346. |
| 40 | Juvonen P. Effects of non-metallic inclusions on fatigue properties of calcium treated steels[D]. Helsinki: Helsinki University of Technology, 2004. |
| 41 | Outka D, Kim Y, Chen A, et al. Method of reducing aluminum fluoride deposits in plasma etch reactor: US6770214B2[P/OL]. 2004[2024-07-15].. |
| 42 | Ramos R, Cunge G, Pelissier B, et al. Cleaning aluminum fluoride coatings from plasma reactor walls in SiCl4/Cl2 plasmas[J]. Plasma Sources Science and Technology, 2007, 16(4): 711-715. |
| 43 | Smith D L, Bruce R H. Si and Al etching and product detection in a plasma beam under ultrahigh vacuum[J]. Journal of The Electrochemical Society, 1982, 129: 2045-2051. |
| 44 | Cunge G, Kogelschatz M, Sadeghi N. Production and loss mechanisms of SiCl x etch products during silicon etching in a high density HBr/Cl2/O2 plasma[J]. Journal of Applied Physics, 2004, 96(8): 4578-4587. |
| 45 | Raoux S, Tanaka T, Bhan M, et al. Remote microwave plasma source for cleaning chemical vapor deposition chambers: technology for reducing global warming gas emissions[J]. Journal of Vacuum Science & Technology B: Microelectronics and Nanometer Structures, 1999, 17(2): 477-485. |
| 46 | Bai B. An experimental study and modeling of transformer coupled toroidal plasma processing of materials[D]. Cambridge: Massachusetts Institute of Technology, 2006. |
| 47 | Kim S B, Seo H, Kim Y, et al. Remote RF oxygen plasma cleaning of the photoresist residue and RIE-related fluorocarbon films[J]. Journal of the Korean Physical Society, 2002, 41(2): 247-250. |
| 48 | Ningel K P, Theirich D, Engemann J. Characterizing the remote plasma polymerization of octafluorocyclobutane induced by RF-driven hollow-cathode discharge[J]. Surface & Coatings Technology, 1998, 98(1/2/3): 1142-1147. |
| 49 | Theirich D, Ningel K P, Engemann J. A novel remote technique for high rate plasma polymerization with radio frequency plasmas[J]. Surface and Coatings Technology, 1996, 87: 628-633. |
| 50 | Wei G. Kinetics modeling and 3-dimensional simulation of surface roughness during plasma etching[D]. Boston: Massachusetts Institute of Technology, 2009. |
| 51 | Li X, Hua X F, Ling L, et al. Surface chemical changes of aluminum during NF3-based plasma processing used for in situ chamber cleaning[J]. Journal of Vacuum Science & Technology A: Vacuum, Surfaces, and Films, 2004, 22(1): 158-164. |
| 52 | So J, Choi E, Kim J T, et al. Improvement of plasma resistance of anodic aluminum-oxide film in sulfuric acid containing cerium(IV) ion[J]. Coatings, 2020, 10(2): 1-10. |
| 53 | Shin J S, Kim M, Song J B, et al. Fluorine plasma corrosion resistance of anodic oxide film depending on electrolyte temperature[J]. Applied Science and Convergence Technology, 2018, 27(1): 9-13. |
| 54 | Vallejo G R, Dayton D D. Ultra high purity electroplated sluminum coatings for critical components in dry etch and process chamber environments[R/OL]. 2015[2024-07-15]. . |
| 55 | Wang Y J, Ma X X, Guo G W. Electrodeposition of aluminum on 316L stainless steel from molten salts based on chlorides[J]. Key Engineering Materials, 2008, 373/374: 273-276. |
| 56 | Maniam K K, Paul S. A review on the electrodeposition of aluminum and aluminum alloys in ionic liquids[J]. Coatings, 2021, 11(1): 1-36. |
| 57 | Zhang M M, Kamavarum V, Reddy R G. New electrolytes for aluminum production: ionic liquids[J]. JOM, 2003, 55(11): 54-57. |
| 58 | Liu Q X, Zein El Abedin S, Endres F. Electrodeposition of nanocrystalline aluminum: breakdown of imidazolium cations modifies the crystal size[J]. Journal of the Electrochemical Society, 2008, 155(5): D357. |
| 59 | Bakkar A, Neubert V. Electrodeposition and corrosion characterisation of micro-and nano-crystalline aluminium from AlCl3/1-ethyl-3-methylimidazolium chloride ionic liquid[J]. Electrochimica Acta, 2013, 103: 211-218. |
| 60 | O’Donnell R J, Daugherty J E. Productivity enhancing thermal sprayed yttria-containing coating for plasma reactor: US20050150866A1[P/OL]. 2005[2024-07-15].. |
| 61 | Lin T K, Wang W K, Huang S Y, et al. Comparison of erosion behavior and particle contamination in mass-production CF4/O2 plasma chambers using Y2O3 and YF3 protective coatings[J]. Nanomaterials, 2017, 7(7): 183-192. |
| 62 | Miwa K, Sawai T, Aoyama M, et al. Particle reduction using Y2O3 material in an etching tool[C]//IEEE International Symposium on Semiconductor Manufacturing Conference Proceedings. Piscataway: IEEE, 2007: 479-482. |
| 63 | Song J B, Kim J T, Oh S G, et al. Contamination particles and plasma etching behavior of atmospheric plasma sprayed Y2O3 and YF3 coatings under NF3 plasma[J]. Coatings, 2019, 9(2): 102-110. |
| 64 | Ashizawa H, Yoshida K. Plasma-resistance evaluation of yttrium oxyfluoride coating prepared by aerosol deposition method[J]. International Journal of Applied Ceramic Technology, 2022, 19: 375-382. |
| 65 | Tai C N, Koh E S, Akari K. Macroparticles on TiN films prepared by the arc ion plating process[J]. Surface and Coatings Technology, 1990, 43: 324-335. |
| 66 | Jun A. Aerosol deposition of ceramic thick films at room temperature: densification mechanism of ceramic layers[J]. Journal of the American Ceramic Society, 2006, 89(6): 1834-1839. |
| 67 | Jun A. Room temperature impact consolidation (RTIC) of fine ceramic powder by aerosol deposition method and applications to microdevices[J]. Journal of Thermal Spray Technology, 2008, 17(2): 181-198. |
| 68 | Ashizawa H, Yoshida K. Investigation of fluoride layer of yttria coatings prepared by aerosol deposition method[J]. Journal of the Ceramic Society of Japan, 2021, 129(1): 46-53. |
| 69 | Le Gouil A, Pargon E, Cunge G, et al. Chemical analysis of deposits formed on the reactor walls during silicon and metal gate etching processes[J]. Journal of Vacuum Science & Technology B: Microelectronics and Nanometer Structures, 2006, 24(5): 2191-2197. |
| 70 | Cunge G, Mori M, Kogelschatz M, et al. Time-resolved measurements of Cl2 density in high-density plasmas and application[J]. Applied Physics Letters, 2006, 88: 051501-051503. |
| 71 | Kim T W, Aydil E S, Soc J E, et al. Effects of chamber wall conditions on Cl concentration and Si etch rate uniformity in plasma etching reactors[J]. Journal of the Electrochemical Society, 2003, 150(7): G418-G427. |
| 72 | Kota G P, Coburn J W, Graves D B. The recombination of chlorine atoms at surfaces[J]. Journal of Vacuum Science & Technology A: Vacuum, Surfaces, and Films, 1998, 16(1): 270-277. |
| 73 | Kota G P, Coburn J W, Graves D B. Heterogeneous recombination of atomic bromine and fluorine[J]. Journal of Vacuum Science & Technology A: Vacuum, Surfaces, and Films, 1999, 17(1): 282-290. |
| 74 | Cunge G, Sadeghi N, Ramos R. Influence of the reactor wall composition on radicals’ densities and total pressure in Cl2 inductively coupled plasmas: II. during silicon etching[J]. Journal of Applied Physics, 2007, 102(9): 1-12. |
| 75 | Cunge G, Sadeghi N, Ramos R. Influence of the reactor wall composition on radicals’ densities and total pressure in Cl2 inductively coupled plasmas: I. without silicon etching[J]. Journal of Applied Physics, 2007, 102(9): 093304-093312. |
| 76 | Xu S, Sun Z, Qian X, et al. Characteristics and mechanism of etch process sensitivity to chamber surface condition[J]. Journal of Vacuum Science & Technology B: Microelectronics and Nanometer Structures, 2001, 19(1): 166-171. |
| [1] | 耿健, 王晓冬, 郭美如, 任正宜. 微型溅射离子泵的阳极筒长度对抽气特性的影响[J]. 东北大学学报(自然科学版), 2023, 44(11): 1596-1603. |
| [2] | 吕江涛, 王筱钧, 鄂思宇, 张亚男. 用于硝酸根浓度测量的反射式光纤SPR传感器[J]. 东北大学学报:自然科学版, 2019, 40(8): 1075-1079. |
| [3] | 沈玲玲, 赵博, 徐君莉, 石忠宁. 等离子体放电电解制备纳米氧化亚铜[J]. 东北大学学报:自然科学版, 2019, 40(5): 668-672. |
| [4] | 闫欣, 王海洋. 金涂覆光子晶体光纤偏振滤波器的特性分析[J]. 东北大学学报:自然科学版, 2018, 39(11): 1540-1544. |
| [5] | 李林敏, 李宝宽, 刘立超, 曹霞. TIG焊流动、传热及界面跟踪动网格数值模拟[J]. 东北大学学报:自然科学版, 2017, 38(10): 1411-1416. |
| [6] | 吕少波;蔺增;巴德纯;李麟涉;. 射频负偏压等离子体鞘层流体动力学模拟[J]. 东北大学学报(自然科学版), 2008, 29(6): 881-884. |
| [7] | 蔺增;巴德纯;王凤;. 含氢类金刚石碳膜的拉曼光谱洛伦兹分解[J]. 东北大学学报(自然科学版), 2007, 28(4): 573-575. |
| [8] | 李明;蔺增;王凤;巴德纯;. 射频PECVD方法生长含氢非晶碳膜的结构及摩擦学性能[J]. 东北大学学报(自然科学版), 2007, 28(12): 1745-1748. |
| [9] | 于撼江;孙凤久;张军;. 用于大气环境中的氮等离子体枪束流模拟[J]. 东北大学学报(自然科学版), 2007, 28(12): 1799-1802. |
| [10] | 刘军友;孙凤久;严平沅;闫伟. 用于大气环境中的氮等离子体枪放电特性及束流强度[J]. 东北大学学报(自然科学版), 2004, 25(2): 201-204. |
| [11] | 张芝涛;鲜于泽;白敏冬;许波. 强电离放电研究[J]. 东北大学学报:自然科学版, 2002, 23(5): 507-510. |
| [12] | 杜晓光. MWP-AAS测定银的某些影响因素的研究[J]. 东北大学学报(自然科学版), 1999, 20(6): 661-664. |
| [13] | -. 80Kev离子注入机[J]. 东北大学学报:自然科学版, 1975, -(4): 39-43. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||